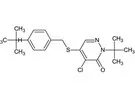
 In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Nissan Chemical Europe S.A.S. submitted a request to the competent national authority in the Netherlands to modify the existing maximum residue levels (MRLs) for the active substance pyridaben in tomatoes and aubergines. An MRL proposal of 0.15 mg/kg was derived for tomatoes and aubergines which reflects the intended use of the plant protection product containing pyridaben. Adequate analytical methods for enforcement are available to control the residues of pyridaben in plant matrices at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concludes that the proposed use of pyridaben on tomatoes and aubergines will not result in a consumer exposure exceeding the toxicological reference values and therefore it is unlikely to pose a risk to consumers’ health.
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Nissan Chemical Europe S.A.S. submitted a request to the competent national authority in the Netherlands to modify the existing maximum residue levels (MRLs) for the active substance pyridaben in tomatoes and aubergines. An MRL proposal of 0.15 mg/kg was derived for tomatoes and aubergines which reflects the intended use of the plant protection product containing pyridaben. Adequate analytical methods for enforcement are available to control the residues of pyridaben in plant matrices at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concludes that the proposed use of pyridaben on tomatoes and aubergines will not result in a consumer exposure exceeding the toxicological reference values and therefore it is unlikely to pose a risk to consumers’ health.
Sign up for our daily Newsletter and stay up to date with all the latest news!
Subscribe I am already a subscriber